Um... yeah, we didn't do a lot with this lab. we looked at tubes with different equilibriums of gases based on temperature. As they grew warmer the levels of the colorless gas decreased, so they grew more and more opaque.
The first, at room temp.
The second, after being heated for all of thirty seconds.
The third, which had been kept in an ice bath (seen below).
These all were shown and the concept of equilibrium was explained with utmost dignity by Sophia and Erica splashing water about the lab area.
Anatomy Understanding is Vital to Art
Wednesday, March 12, 2014
Sunday, March 9, 2014
Three Questions.
What have you completed recently?
I wish I could say I did a lab Thursday learning about rate reactions, but due to a series of events, at the time of the lab I was standing outside Onate High School in full costume for Beauty and the Beast, during a fire drill that hit right in the middle of Gaston. It was tiring, to say the least. What I have completed recently is portraying the role of Madame de la Grand Bouche (the grammatically incorrect wardrobe in Beauty and the Beast) five times over the course of three days.
What have you learned recently?
I have learned about reaction rates, I have learned I have a wicked (in a good way) shakedown technique, and I have learned that the patch of skin underneath the lower eyelid is really tender if you rub it too much over the course of five days.
What are you planning on doing next?
I plan on pulling my grades back up from where they might have fallen, and to visit colleges over spring break.
I wish I could say I did a lab Thursday learning about rate reactions, but due to a series of events, at the time of the lab I was standing outside Onate High School in full costume for Beauty and the Beast, during a fire drill that hit right in the middle of Gaston. It was tiring, to say the least. What I have completed recently is portraying the role of Madame de la Grand Bouche (the grammatically incorrect wardrobe in Beauty and the Beast) five times over the course of three days.
What have you learned recently?
I have learned about reaction rates, I have learned I have a wicked (in a good way) shakedown technique, and I have learned that the patch of skin underneath the lower eyelid is really tender if you rub it too much over the course of five days.
What are you planning on doing next?
I plan on pulling my grades back up from where they might have fallen, and to visit colleges over spring break.
Friday, February 21, 2014
Three questions blog... I should really stop procastinating on these...
What have I learned lately?
I have learned about hybridization, I have leanred more specific qualities and properties of the three main types of compounds (ionic, covalent and metallic).
What have I done lately?
I have gotten sick and taken notes. My life is just flush with activity.
What do I hope to do?
Finish getting caught up on things i missed due to my wisdom teeth. Find time to just marinate in laziness. Take up scuba diving. Introduce folk dance to the community. And pass the test coming up on Monday so that I can keep my grade at at least a C so come April I can go to a Cage the Elephant concert.
I have learned about hybridization, I have leanred more specific qualities and properties of the three main types of compounds (ionic, covalent and metallic).
What have I done lately?
I have gotten sick and taken notes. My life is just flush with activity.
What do I hope to do?
Finish getting caught up on things i missed due to my wisdom teeth. Find time to just marinate in laziness. Take up scuba diving. Introduce folk dance to the community. And pass the test coming up on Monday so that I can keep my grade at at least a C so come April I can go to a Cage the Elephant concert.
Monday, February 17, 2014
Classification of solids
...Labs are fun!
Anyway, the most recent lab, Classification of Solids, meant burning innocent test tubes- I mean melting the substances inside the test tubes!- seeing if they were soluble, and seeing if they could conduct electricity. The questions related to this lab were mostly interpreting the results and actual questions relating to the knowledge acquired during the lab.
I suppose I should answer those.
An ionic solid would be soluble in water and conduct electricity in the melt, a network solid would be soluble in organic solvents, a metal would conduct electricity when under pressure (or at just about any other time), and a molecular solid would be slightly soluble in water and slightly conductive in said solution. If a solid was white at room temp, melted at 80 degrees Celsius and the melt had slight electrical conductivity it would be an ionic solid because it is presumably crystalline and conducts electricity. If a white solid melted at 1000 degrees Celsius and was insoluble in all solvents, and didn't conduct electricity at all, it would be an inorganic network solid. Potassium is a metal, Calcium Carbonate is an inorganic network solid, Octane is a molecular solid, and Hydrogen Chloride is a molecular solid as well. Speaking generally, metals are insoluble in water, network solids are ductile and malleable, and about anything but molecular solids are nonvolatile. A test that didn't work well in this lab was the melting portion of the lab because there wasn't enough fire. But really, the lab was very well produced all-around. If I had one complaint, it would be the substances chosen. Some portions there was little to no variety and in others there were no defining factors between substances, even when just looking at them (cobalt chloride excluded).
Night y'all!
Anyway, the most recent lab, Classification of Solids, meant burning innocent test tubes- I mean melting the substances inside the test tubes!- seeing if they were soluble, and seeing if they could conduct electricity. The questions related to this lab were mostly interpreting the results and actual questions relating to the knowledge acquired during the lab.
I suppose I should answer those.
An ionic solid would be soluble in water and conduct electricity in the melt, a network solid would be soluble in organic solvents, a metal would conduct electricity when under pressure (or at just about any other time), and a molecular solid would be slightly soluble in water and slightly conductive in said solution. If a solid was white at room temp, melted at 80 degrees Celsius and the melt had slight electrical conductivity it would be an ionic solid because it is presumably crystalline and conducts electricity. If a white solid melted at 1000 degrees Celsius and was insoluble in all solvents, and didn't conduct electricity at all, it would be an inorganic network solid. Potassium is a metal, Calcium Carbonate is an inorganic network solid, Octane is a molecular solid, and Hydrogen Chloride is a molecular solid as well. Speaking generally, metals are insoluble in water, network solids are ductile and malleable, and about anything but molecular solids are nonvolatile. A test that didn't work well in this lab was the melting portion of the lab because there wasn't enough fire. But really, the lab was very well produced all-around. If I had one complaint, it would be the substances chosen. Some portions there was little to no variety and in others there were no defining factors between substances, even when just looking at them (cobalt chloride excluded).
Night y'all!
Wednesday, January 29, 2014
Soap is repulsive...
When mixed with water and pepper anyway...
So, what I'm basically saying is that soap spreads on the surface of the water due to the fact that it has both polar and nonpolar poles. This pushes the pepper to the edges. Observe below:
BEHOLD!
So yeah soap is repulsive to pepper. When it's just water and soap, the soap gently floats to the bottom of the cup and then mixes in of sorts.
So, what I'm basically saying is that soap spreads on the surface of the water due to the fact that it has both polar and nonpolar poles. This pushes the pepper to the edges. Observe below:
So yeah soap is repulsive to pepper. When it's just water and soap, the soap gently floats to the bottom of the cup and then mixes in of sorts.
The yellow tint around the edges is the soap.
Sunday, January 26, 2014
Three Questions: The Sequel of the sequel of the sequel of the sequel to the Nth power
What have I done or learned lately? *Inhales deeply for long exposition* ....I got nothin. I could say I learned I'm horrible at taking tests, and that I love Celtic music, but I kind of knew that before. I have been working with the theater on stuff for the Beauty and the Beast musical (performances are in Mid-March, more details on the way) but, being cast as the wardrobe, I just stand there and recite lines with my arms at uncomfortable angles. Honestly, I can hardly even walk in the darn costume. Oh and I'm too tall for it, which means I crouch over more than the Hunchback of Notre Dame.
What am I going to do? Go to the library sometime in the near future and return books. Teach myself how to play the bagpipes and figure skate simultaneously. Take over the world. Finish test corrections and do online Government assignments. And hopefully not spontaneously combust.
Have a nice day everybody!
What am I going to do? Go to the library sometime in the near future and return books. Teach myself how to play the bagpipes and figure skate simultaneously. Take over the world. Finish test corrections and do online Government assignments. And hopefully not spontaneously combust.
Have a nice day everybody!
Monday, January 20, 2014
GRAS: Generally Regarded as Satirical
I kid, I kid. GRAS stands for "Generally Regarded as Safe."
On an absolutely unrelated note which will in no way connect to anything later, there are seven basic food dyes: FD&C Blue No. 1 & 2 (Brilliant Blue FCF and Indigotine), FD&C Green No. 3 (Fast Green FCF), FD&C Red No. 40 & 3 (Allura Red AC and Erythrosine), FD&C Yellow No. 5 & 6 (Tartazine and Sunset Yellow FCF), and Chanel No. 05 (Aubazine).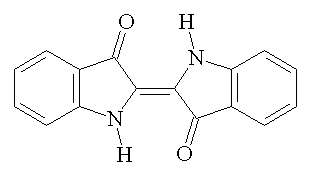 |
| FD&C Blue No. 2 from www.chm.bris.ac.uk |
 |
| FD&C Blue No. 1 from medicinescomplete.com |
 |
| FD&C Green No. 3 from wikipedia |
 |
| FD&C Red No. 40, also from wikipedia |
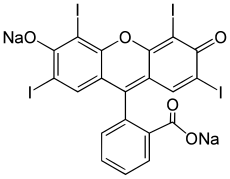 |
| FD&C Red No. 3, again from wikipedia |
 |
| FD&C Yellow No. 5... from (all together now,) wikipedia |
 |
| FD&C Yellow No. 6, from thearubigin.blogspot.com for a change. |
 |
| Chanel No. 5 from smithandbrandon.wordpress.com because I could. Also Aubazine is a convent where Coco Chanel lived in her teenage years with nuns. |
Subscribe to:
Posts (Atom)



