I kid, I kid. GRAS stands for "Generally Regarded as Safe."
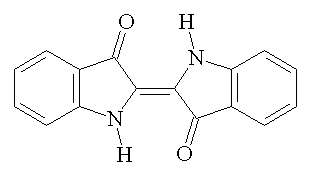
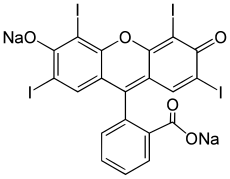
On an absolutely unrelated note which will in no way connect to anything later, there are seven basic food dyes: FD&C Blue No. 1 & 2 (Brilliant Blue FCF and Indigotine), FD&C Green No. 3 (Fast Green FCF), FD&C Red No. 40 & 3 (Allura Red AC and Erythrosine), FD&C Yellow No. 5 & 6 (Tartazine and Sunset Yellow FCF), and Chanel No. 05 (Aubazine).
GRAS standards classify food dyes as: "...any dye, pigment, or other substance that can impart color to a food, drug, or cosmetic or to the human body." (http://www.fda.gov/ForIndustry/ColorAdditives/RegulatoryProcessHistoricalPerspectives/#) It is regulated by individual regulations for each dye. Products that don't comply are subject to enforcement action (recalls, legal actions, etc.), even for only a small amount. The seven food dyes mentioned before (which still don't pertain to anything) are okay-ed by the FDA for use in "Foods generally." (
http://www.fda.gov/ForIndustry/ColorAdditives/RegulatoryProcessHistoricalPerspectives/#) The history of dyes is a rich one, dating back to ancient Rome when dyes for clothing and cosmetics were collected from various plants and, in the case of Tyrian purple (named for the city of Tyre where it was first recorded), shellfish. The celts around the same time period again used plants for war paint and fabric dye. The ancient chinese used vibrant silks as a status symbol, but the poor were mostly stuck with browns and greens, in any culture. Dyes were then used in clothing and cosmetics constantly. In 1856, William Henry Perkin discovered the first synthetic dye, and orange color he called mauve (otherwise known as aniline). (
http://www.straw.com/sig/dyehist.html) Federal oversight of dyes began in the 1880s, and in 1960 the
Color Additives Amendments, later known as GRAS, were put in place. These stated that dyes would be put on a provinional list and only placed on a permanant list when scientifically deemed safe dor human use or comsumption. (
http://www.fda.gov/ForIndustry/ColorAdditives/RegulatoryProcessHistoricalPerspectives/#) The GRAS regulations pertain to food as they are responsible for what goes into an average American's meal.
 |
| FD&C Yellow No. 5... from (all together now,) wikipedia |
 |
Chanel No. 5 from smithandbrandon.wordpress.com because I could.
Also Aubazine is a convent where Coco Chanel lived in her teenage years with nuns. |